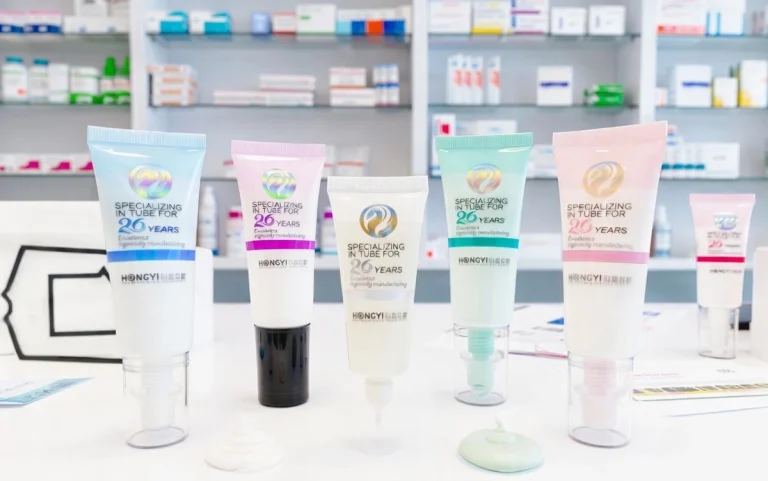
As a specialist in the cosmetic packaging engineering sector since 2009, Hongyi Plastic recognizes that for a packaging engineer, quality is never a marketing buzzword—it is a set of verifiable parameters. We operate a 3,000-square-meter high-precision manufacturing base, where our 100,000-grade GMPC cleanroom is designed to mitigate microbial risks at the molecular level. With a daily capacity of approximately 100,000 tubes and dual ISO9001/ISO45001 certifications, we provide a robust technical infrastructure for high-standard brand launches. At Hongyi Plastic, we believe a tube is not merely a container; it is a calibrated delivery system that must maintain structural and biological integrity from the production line to the final user application.
The Strategic Importance of Packaging Integrity in Global Logistics
In world supply chain work, sea shipping boxes are risky spots. Container rain happens. It is water from big day-night temp changes. This puts hard pressure on packaging. The risk grows fast for the plush powder puff design plastic tube bottle. The soft material in these tools has a lot of surface for its size. So, it pulls in water easily.
From an engineering view, tiny water on rough spots feeds fungus well. If germs get in during a 30-45 day trip, the whole batch is bad. For a packaging engineer, this is more than lost goods. It hurts the brand name. It also costs a lot to call back items. So, you need good Water Vapor Transmission Rate (WVTR) control. You also need low starting germ levels. These keep global safety rules. Past lost stock, other risks come. Fungus waste can change the formula’s pH and active strength. This hits high-value cosmetics over time. We focus on these to keep your products safe in long trips.
Technical Deep Dive: The Precision Engineering of Plush Puff Tubes
Stable packaging starts with right shape picks and material choices. The φ19 small-diameter design is a smart pick. It fits thick local formulas like color fixers, eye liquids, or medicated creams. Its small build cuts empty inside space. This lowers trapped air after filling. So, it cuts water buildup chance. By setting content to empty space well, we drop oxygen pressure inside. This key step stops air-breathing germs.
In material work, block power is the main focus of our R&D. We use good PE or ABL (Aluminum Barrier Laminate) builds. We get raw parts from top sellers in Qatar, Brazil, and Japan. These pass checks for low leak and no chemical change. The steady wall stops oxygen and water move. So, the inside stays safe from mold seeds. This holds even if outside Relative Humidity (RH) jumps in travel. Our tests include fast age checks at 40°C and 75% RH. They copy hard ocean trips. This makes sure the block stays good for the full shelf life. We do this to give you reliable tubes every time.
Manufacturing Excellence: Eliminating Contamination at the Source
Germ control must be part of daily work rules (SOPs). Normal factory floors do not fit clean soft tool needs. We make in GMPC-checked spots. Air quality stays tight with HEPA filters. We keep steady positive pressure (at least 10-15 Pa over other areas). This setup blocks fungus seeds in the build step.
To cut people risks more, we use full auto lines. In our special cap and soft rooms, puff tops go on and seal by machines. This no-touch way stops dirt from humans. Things like skin oils or germs feed mold. Each batch gets close checks in our set system. We use auto light scans (AOI) to spot tiny flaws on the soft part. Only low-germ units go to final pack. This control keeps germ count near zero at seal time. It makes formula protectors work less hard. Our full steps ensure clean starts. This helps avoid mold in travel or hold. We meet world needs for safe items with these practices.
Advanced Anti-Mold Strategies for Shipping and Storage
Past main packaging, world shipping needs extra guards. The colors and coats on the tube are UV-set and checked for steady chemicals in wet tests. So, the outer layer does not break or feed fungus in bad spots. We also check how the soft glue works with the formula. No bad vapors come out in storage. These could start germ growth.
For extra packaging, we suggest medical desiccants in big boxes. They cut inside RH. We have a hold area in our 1,000+ square meter set warehouse. Goods settle in checked air before send. This shift care is key. It keeps the soft fibers dry and strong. They stand up to ocean trip changes. Our warehouse system (WMS) uses First-In, First-Out (FIFO). It stops harm from long still storage in bad setups. These steps lower mold odds. They protect your goods end to end.
Engineering Support and Supply Chain Efficiency
Seller trust matters as much as tech papers. We give a 12-month quality promise on all sent items. If shape fails or germs show from make errors, we have a clear swap plan. This cuts money and work risks for big buys. Our Quality Assurance (QA) team keeps full track records. They cover raw part papers (COA) to end checks.
Our R&D group has over 100 mold sets and 20+ patents. This lets us do quick models (1-3 days) and big runs (5-20 days). This speed helps engineers test steady and fit early. It shortens time to market. It meets all needs, like PCR (Post-Consumer Recycled) fit, without losing guard roles. We help teams with Drop Tests and Leakage Tests in low air. These check if the φ19 seal holds in plane pressure shifts. Our support makes your chain run smooth and safe.
Technical Services for Professional Applications
Our tubes work well in cosmetics. But their strong block and anti-mold traits fit pro drug and work uses too. For eye liquids needing clean put-on or gels for spot delivery, our team gives TDS (Technical Data Sheets) and rule papers. We have sent packs for spot acne care and scar fix gels. There, germ clean meets medical rules.We add digital track systems for clear chain views. Packaging engineers can watch travel time and air for parts. By mixing ISO make with good material know-how, we give packs that hit top world rules for safe work. Our consult help covers better fill ways. It makes sure the tool and formula link is solid. This full service aids pro needs well.
For technical documentation, TDS requests, or to order a sample kit for compatibility testing, please contact us.
FAQ
Q: What specific technical measures ensure the flocking material does not accumulate condensation?
A: We set fiber closeness right. We pick man-made polymers for soft parts. They hold less water than real fibers. With RH watch in the GMPC room, positive pressure, and desiccant in extra pack, we keep top water under mold start level (often below 60% inside RH). This keeps things dry and safe.
Q: How do you verify the barrier performance of the φ19 tubes?
A: We run standard WVTR (Water Vapor Transmission Rate) and OTR (Oxygen Transmission Rate) tests by ASTM rules. High-weight materials from top world sellers give steady build. They block well for long shelf items. We also do Real-time Stability Tests and Accelerated Stability Tests. These check block strength over 12-24 months.
Q: Can these tubes be used for formulas containing Active Pharmaceutical Ingredients (APIs)?
A: Yes. Our PE and ABL parts have high no-react chemicals. They fit many active parts. We give full MSDS (Material Safety Data Sheets), resist reports, and fit test help. This makes sure no bad mixes between formula and tool. It meets safe rules for medical and drug packs.